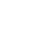Nankai Team's Findings on the Mechanism of Anti-tuberculosis Drug Bedaquiline (BDQ) and Its Derivatives Published in Nature

Recently, the top international academic journal Nature published online the latest research results of Professor Gong Hongri and Academician Rao Zihe from the College of Life Sciences, Nankai University, and other collaborators. This research elucidates the molecular mechanism by which BDQ and its analogue TBAJ-587 inhibit the ATP synthase of Mycobacterium tuberculosis, and reveals the mechanism of their cross-reaction with ATP synthase in humans, which is of great significance for the development of new generation of highly selective drugs for tuberculosis.

On July 8, these results were presented in a press conference held at the Shiing-Shen Building, Balitai campus, Nankai University. Yang Qingshan, Chancellor of Nankai University, and Rao Zihe, CAS academician and former President of Nankai University, attended the conference and delivered speeches. Zhong Nanshan, CAE academician and Director of the Guangzhou National Laboratory, delivered a written speech. About 120 people, including Shen Zhongyang, former president of Tianjin First Central Hospital, Yao Zhi, former Chancellor of Tianjin Medical University, Li Liang, Vice President of Beijing Chest Hospital and former Chairman of the Chinese Society for Tuberculosis of the Chinese Medical Association, Guan Minxin, Director of the Institute of Genetics and former director of the College of Life Sciences of Zhejiang University, Bi Lijun, a principal investigator of the Guangzhou National Laboratory, as well as representatives of central and local mainstream media, faculty and students of Nankai University, were there to witness this important milestone in scientific research.
Academician Zhong Nanshan said in his written speech that the breakthrough achieved by Professor Gong Hongri, Academician Rao Zihe and their team in the field of anti-tuberculosis drugs not only consolidated the basis for cutting-edge theoretical research in the field of tuberculosis, but also provided more possibilities for designing anti-tuberculosis drugs with higher selectivity. This was a useful practice under the new system for mobilizing the resources nationwide. The Guangzhou National Laboratory would continue to assist the research team in finding anti-tuberculosis drugs with good clinical efficacy, less side effects, and better safety, based on this achievement, in order to realize the key goal of improving people's health.
Tuberculosis is an infectious disease caused by Mycobacterium tuberculosis, which mainly attacks the lungs and can lead to death in serious cases, so being one of the major public health problems of global concern. BDQ, an inhibitor of Mycobacterium tuberculosis ATP synthase, can effectively inhibit the growth of Mycobacterium tuberculosis. As the first new anti-tuberculosis drug developed globally and launched in nearly 50 years, BDQ has been listed by the World Health Organization as the first-choice drug in the long-term treatment of tuberculosis resistant to rifampicin or multiple drugs.
However, studies have found that BDQ increases the risk of cardiac arrhythmias in patients due to its interaction with the hERG potassium ion channel, in addition to having potential cross-inhibitory activity on human ATP synthase. Therefore, revealing the molecular mechanisms how the ATP synthase of Mycobacterium tuberculosis works, and how BDQ inhibits Mycobacterium tuberculosis and human ATP synthase, is of great significance for the development of novel inhibitors for Mycobacterium tuberculosis ATP synthase.

Using the gene knock-in/knock-out & gene overexpression strategy in combination with affinity chromatography and gel filtration chromatography (GFC), the research team purified homogeneous, stable and active Mycobacterium tuberculosis ATP synthase from Mycobacterium smegmatis.
After optimizing the conditions for preparing frozen samples, the research team obtained the high-resolution cryo-electron microscopy structure of Mycobacterium tuberculosis ATP synthase bound to BDQ (Figure 1). They found that BDQ strongly interacts with Mycobacterium tuberculosis ATP synthase through quinolinyl (A unit) and dimethylamino (D unit) and binds to multiple sites in the transmembrane domain, preventing the rotation of the c-ring in the transmembrane domain of ATP synthase, blocking proton transport, preventing ATP synthesis, and achieving the goal of starving Mycobacterium tuberculosis to death.
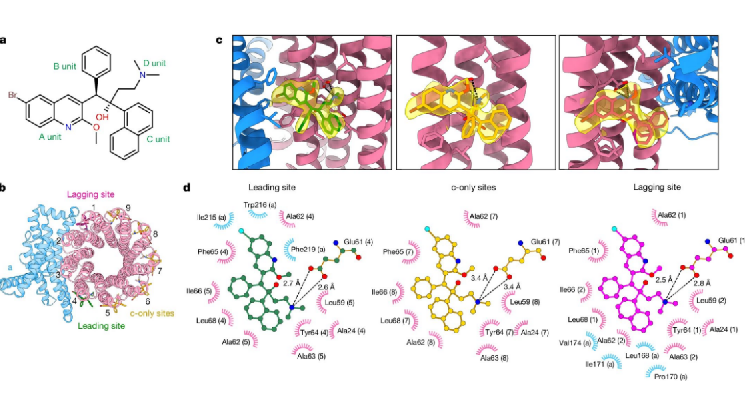
Figure 1. Cryo-electron microscopy structure of Mycobacterium tuberculosis ATP synthase bound to BDQ
Of BDQ derivatives, the most representatives are TBAJ-587 and TBAJ-876. Currently, candidate drugs have entered clinical trials. The research team resolved the high-resolution cryo-electron microscopy structure of Mycobacterium tuberculosis ATP synthase bound to TBAJ-587 (Figure 2). The structure shows that the binding mode of TBAJ-587 to Mycobacterium tuberculosis ATP synthase is the same as that of BDQ. Moreover, TBAJ-587 and BDQ mainly interact with Mycobacterium tuberculosis ATP synthase through A and D units.
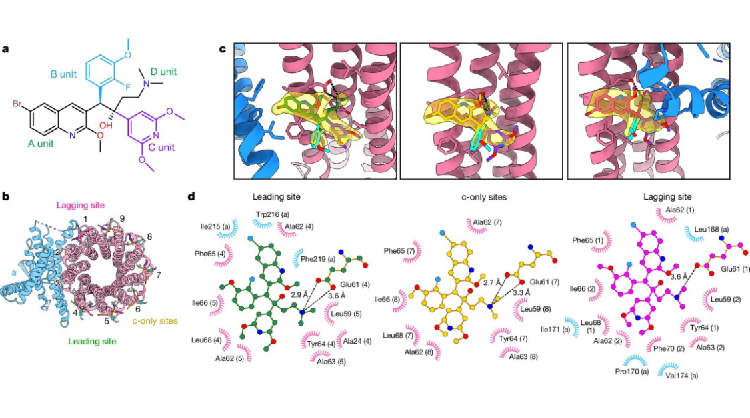
Figure 2. Cryo-electron microscopy structure of Mycobacterium tuberculosis ATP synthase bound to TBAJ-587
Through analyzing the structures, researchers found that both BDQ and TBAJ-587 have cross-reactivity to human ATP synthase. They also resolved the cryo-electron microscopy structure of human ATP synthase bound to BDQ (Figure 3), and found that the redesigned B and C units in TBAJ-587 only reduces the risk of cardiac arrhythmias caused by the interaction with hERG protein. Only optimization of the A unit may reduce the interaction with human ATP synthase, thereby avoiding potential risks in clinical treatment.

Figure 3. Cryo-electron microscopy structure of human ATP synthase bound to BDQ
It is worth mentioning that Nature invited Professor Gregory Cook, former chairman of the Gordon Conference on Bioenergetics well-known in the international academic community, and his colleagues to write a comment on this study in the News & Views column, entitled Blueprints for ATP Machinery Will Aid Tuberculosis Drug Design.

Professor Gong Hongri said that the research results were of great significance for both basic research and clinical translation, and would promote further optimization of BDQ and development of more-effective drugs.

Academician Rao Zihe said, Our team has long been committed to the research of the structure and function of proteins related to pathogens of new and recurrent infectious diseases, as well as the research & development of innovative drugs. We have currently initiated the research on new inhibitors for Mycobacterium tuberculosis ATP synthase, striving to develop new anti-tuberculosis drugs with independent intellectual property rights as soon as possible.

Zhang Yuying and Lai Yuezheng, both of whom are bachelor-straight-to-doctorate students at Nankai University, are co-first authors of this article. Professor Gong Hongri and Academician Rao Zihe from the College of Life Sciences at Nankai University, Associate Researcher Liu Fengjiang from the Guangzhou Laboratory, and Associate Researcher Gao Yan from Shanghai Institute for Advanced Immunochemical Studies at Shanghai Tech University, are co-corresponding authors. Nankai University is the first affiliation of this article.

Paper Link:
https://www.nature.com/articles/s41586-024-07605-8